Background: Acute myeloid leukemia (AML) is one of the most highly refractory hematologic malignancies despite intensive combination chemotherapy and bone marrow stem cell transplantation. Lack of curative treatments is in large part due to our poor understanding of the disease biology and paucity of therapeutic targets. In an effort to identify actionable targets, we recently completed the largest genome, epigenome and transcriptome profiling of AML in nearly 3000 children and young adults. This discovery effort has led to the identification of a library of novel AML-restricted targets (high expression in AML, minimal-to-no expression in normal hematopoiesis) for therapeutic development. One such target was MSLN which encodes for mesothelin, a cell surface adhesion molecule that is highly expressed in 30-50% of AML cases in pediatric (Children Oncology Group) and adult (MD Anderson) cohorts and is entirely absent in normal bone marrow and peripheral blood CD34+ cells. MSLN expression in normal tissues is confined to mesothelial cells lining the pleura, pericardium, and peritoneum. Previous studies targeting MSLN in solid tumors have demonstrated clinical efficacy with minimal toxicities. Given that T cells genetically modified to express chimeric antigen receptors (CARs) are extremely effective at eradicating relapsed and refractory malignancy, we developed MSLN-directed CAR T cells for pre-clinical evaluation in AML.
Methods: From primary patient samples, we verified MSLN expression by RT-PCR and confirmed mesothelin surface protein expression on leukemic blasts by flow-cytometry as well as detected soluble mesothelin in the plasma by ELISA. The VH and VL sequences from Amatuximab were used to create the scFv domain of the standard CAR (41-BB and CD3Zeta). For in vivo CAR T study, Nomo-1 cells, which express endogenous level of MSLN, and Kasumi-1 cells engineered to express MSLN with a lentivirus construct (Kasumi-1 MSLN+) were transplanted into NSG mice. Mock transduced MSLN-directed CAR T cells were infused 1 week (Nomo-1) and 2 weeks (Kasumi-1 MSLN+) following leukemic cell injection. Leukemic burden was measured by bioluminescence IVIS imaging weekly. For in vitro study, Nomo-1 cells were treated with GM6001 (50uM), a metalloprotease inhibitor, or DMSO control for 48 hr prior to evaluation of surface mesothelin by flow cytometry and soluble mesothelin in the culture supernatant by ELISA.
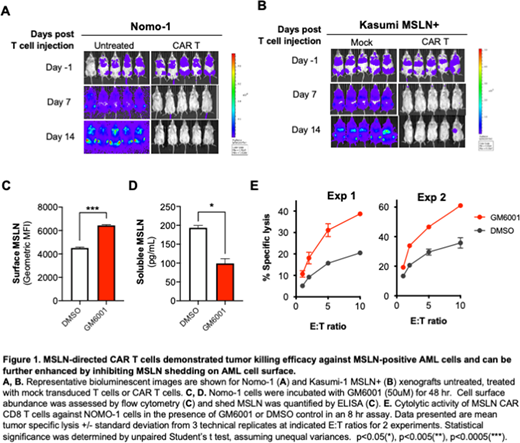
Results:In vivo cytotoxicity of CAR T cells against Nomo-1 and Kasumi-1MSLN+ AML models demonstrated potent, target-dependent tumor killing. After 1- and 2-weeks post CAR T infusion, leukemic cells were eradicated in both Nomo-1 (p<0.0005, week 2, Figure 1A) and Kasumi-1 MSLN+ xenografts (p<0.005 at week 2, Figure 1B). Mesothelin undergoes shedding at the cell membrane as a result of ADAM17-mediated cleavage. Blocking ADAM17 activity with GM6001 in Nomo-1 cells led to increased cell surface mesothelin (Figure 1C) with a corresponding reduction in the shed form (Figure 1D), suggesting that GM6001 treatment stabilizes mesothelin on the cell surface. Furthermore, GM6001 treatment during co-culture of Nomo-1 and CAR T cells enhanced cytolytic activity of CAR T cells (Figure 1E). GM6001 treatment did not significantly impact cell viability of Nomo-1 cells in the absence of CAR T cells (data not shown).
Conclusion: In this study, we demonstrate that mesothelin is a viable therapeutic target and a potential diagnostic biomarker in AML. We show that MSLN CAR T cells were highly effective in eliminating MSLN-positive AML cells in vitro and in vivo. Shedding contributes to the loss of mesothelin antigen and provides a source of soluble mesothelin that may interfere with antibody-based therapies, including CAR T cells. Modulating MSLN shedding by inhibiting ADAM17-mediated cleavage resulted in stabilized mesothelin and improved CAR T cell functionality. This work warrants further evaluation of MSLN CAR T cells to be tested in clinical trials for AML and demonstrates that inhibiting MSLN shedding is a promising approach to improve CAR T efficacy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal